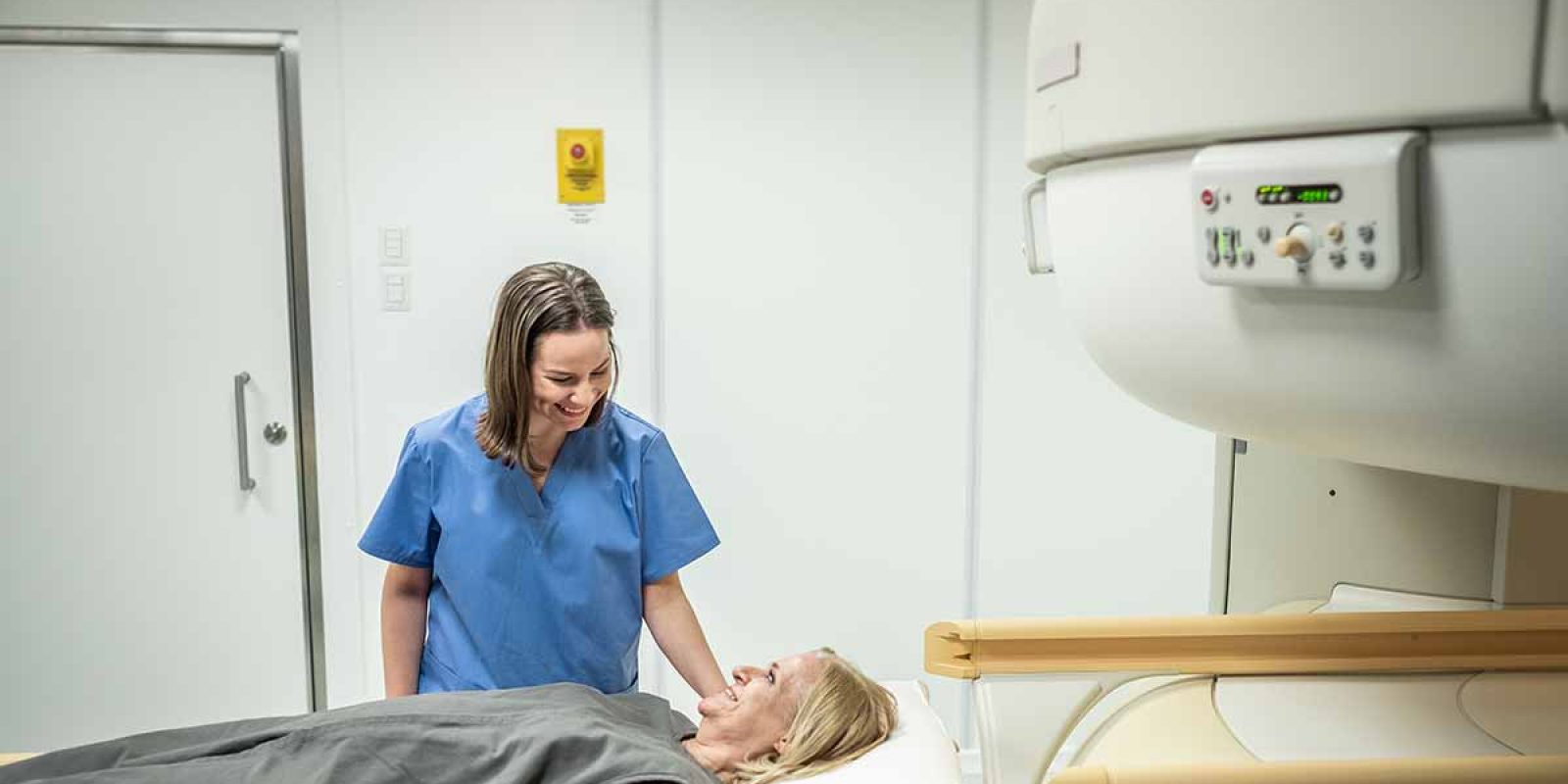
Informing Cancer Patients About Next Steps After Breast Implants
Dr. Libby Copeland-Halperin, a diplomate of the American Board of Surgery and a candidate member of the American Society of Plastic Surgeons discusses a new study that shows breast cancer patients who received silicone implants face challenges in following important FDA recommendations. According to the research, only a small number of the respondents (5.9%) had undergone an MRI screening in accordance with the FDA Recommendations. She talks about access/barriers to care, the importance of screening, and why insurance companies should cover the cost.
Dr. Libby R. Copeland-Halperin is board-certified in general surgery and board-eligible in plastic and reconstructive surgery. She is a diplomate of the American Board of Surgery and a candidate member of the American Society of Plastic Surgeons. Dr. Copeland-Halperin has over 70 presentations and publications and has presented her research at regional and national scientific meetings.
Dr. Copeland-Halperin completed her undergraduate studies at Williams College and medical school at the Icahn School of Medicine at Mount Sinai in New York. After receiving her medical degree, Dr. Copeland-Halperin completed an internship and residency in general surgery at the Inova Fairfax Medical Campus, a large tertiary care hospital in Northern Virginia, where she became Chief Resident for Quality and Safety and served on several hospital committees. After training in general surgery, Dr. Copeland-Halperin pursued additional training in plastic and reconstructive surgery at Dartmouth-Hitchcock Medical Center. She then pursued additional advanced training in microsurgery with a focus on implant-based and autologous breast reconstruction at the Brigham and Women’s Hospital in Boston. Dr. Copeland-Halperin is in private practice at Michelle Copeland DMD MD FACS PC in New York City and is affiliated with Lenox Hill Hospital, an affiliate of Northwell Health.